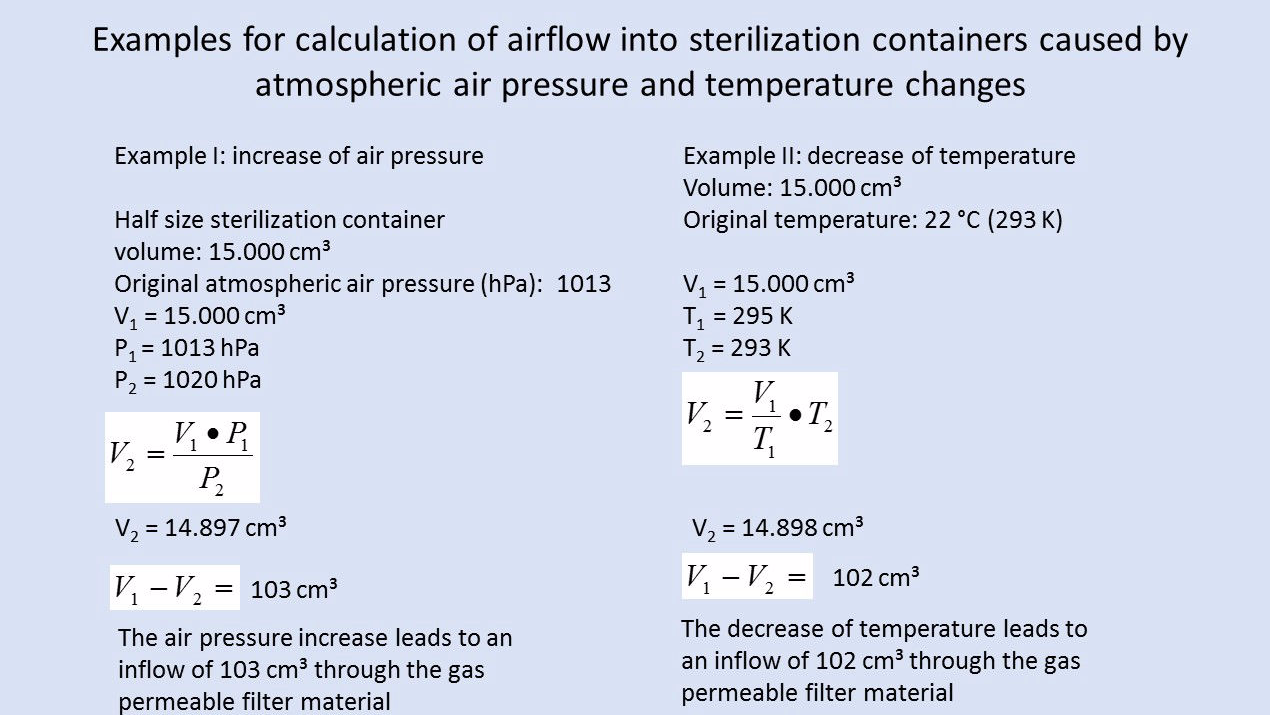
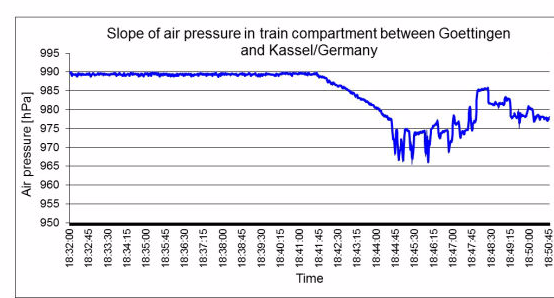
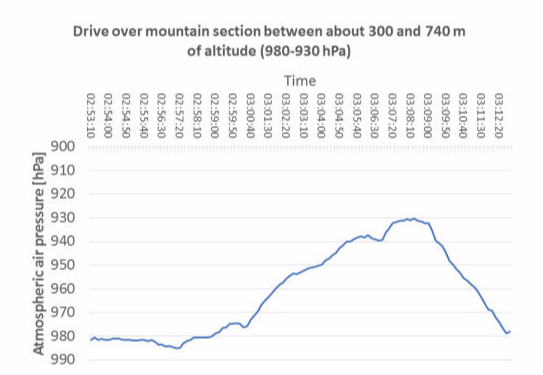
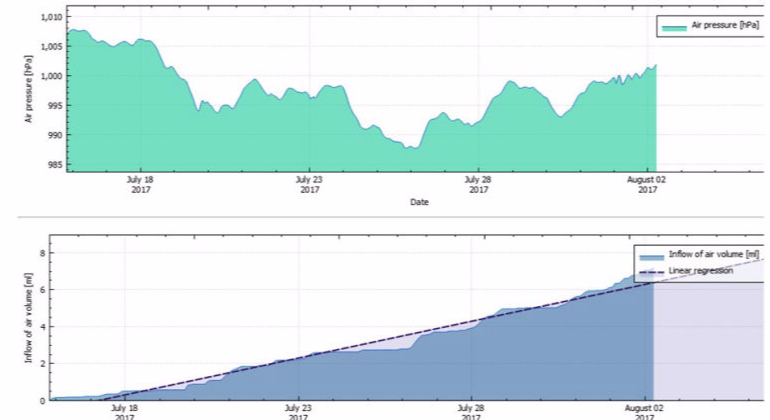
Exposure to different physical factors (chiefly meteorological changes in air pressure, transport to locations at different altitudes and changes in temperature) lead to an exchange of air between the inside of the sterilized packaging and the surroundings. The volume of the gas mass within sterilized packages varies dependently on temperature and air pressure changes. An increase of air pressure and a decrease in temperature reduce the gas volume, and vice versa. Airflows through the porous part of the packaging are the result when the packaging volume is constant.
In terms of physics, the volume flow of air into the packaging accords with the Boyle-Mariotte law "p x V = constant" when air pressure changes are given. According to the first Gay-Lussac law, the temperature-dependent volume change is determined for the isobaric states using the thermal volume-expansion coefficient. The determined or estimated airborne microbial concentration is also required for calculation of the airborne microbial impact. The inflow of environmental air through the packaging material in the period of storage and transport should be based on quantitative risk analysis.